Dry Eye is more than an inconvenience. Dry Eye is a disease.
Many people suffer for years from the discomfort and pain of Dry Eye Disease Most try various products to manage their disease, and while many of the Over-The-Counter products can provide relief, it is just temporary symptom relief.
Meibomian Gland Dysfunction (MGD) is a leading cause of Dry Eye Disease.1 Dry Eye Disease is rarely due to a lack of the watery part of your tears. 86% of dry eye patients have MGD.2
What is Meibomian Gland Disfunction (MGD)
Meibomian Gland Dysfunction is a progressive disease of the ocular surface. Long term, failure to treat MGD can lead to chronic discomfort and degradation of vision, significantly impacting quality of life.3
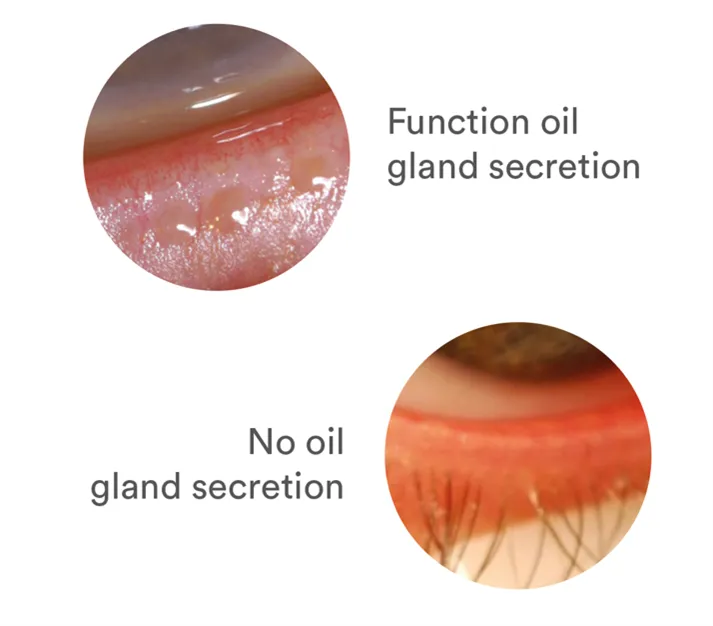
Healthy Meibomian glands that line your upper and lower eyelids secrete oil with every blink. Your Meibomian glands produce the oily part of the tear film needed to protect the surface of your eye by preventing the evaporation of the watery part of your tears. When this function is not working well, your eyes may feel dry. Keeping the function and structure of your Meibomian glands healthy before you become symptomatic is key, as MGD progresses over time.
What causes MGD?
MGD is caused by anatomical changes in the Meibomian glands If left untreated, MGD can become progressively worse over time.
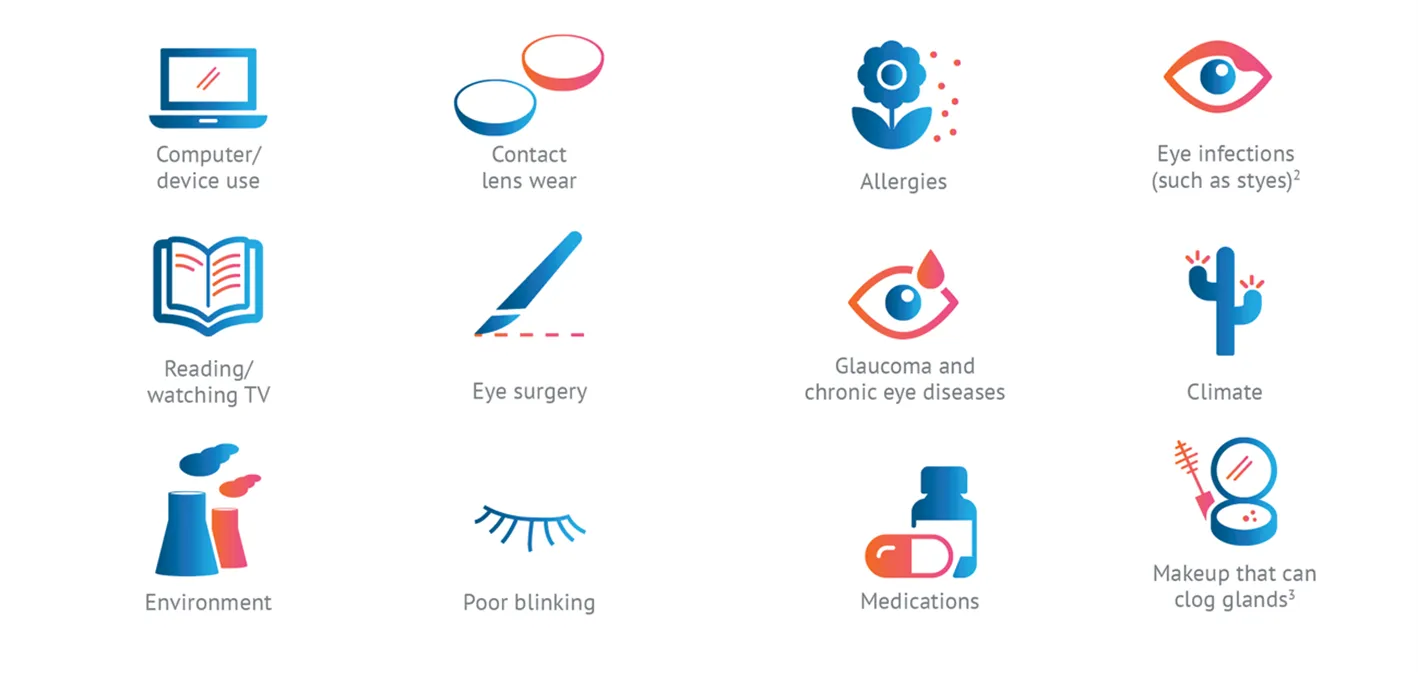
Conditions that can contribute to MGD:
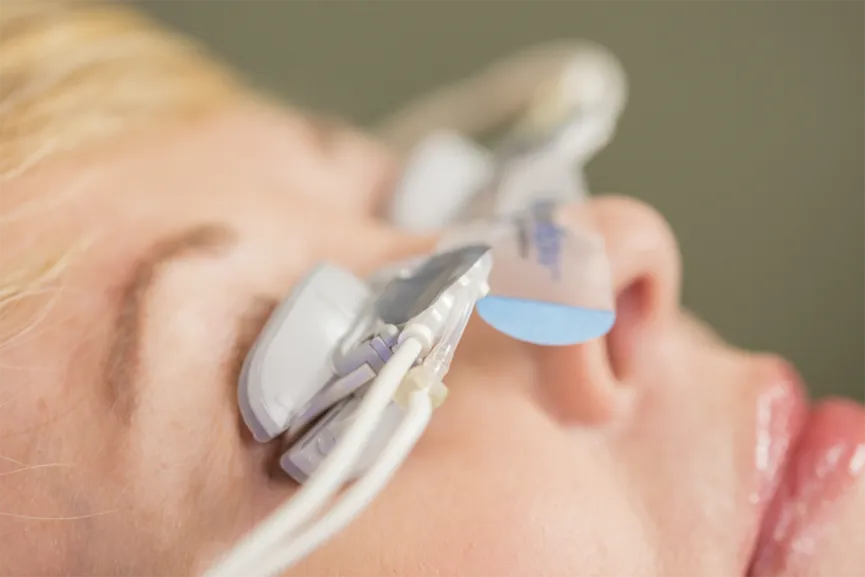
Dry Eyes? Don’t just treat your symptoms, Treat the Cause and MOVE FORWARD with MGD treatment with TearScience® LipiFlow®
For patients with MGD, TearScience® LipiFlow® significantly improves gland secretions and ocular surface symptoms including dry eye and less frequent or blurry vision.4
- The treatment is non-invasive procedure performed in the comfort of your doctor’s office, only takes 12 minutes and can have long-lasting results5
- After initial anesthetic drops, no drugs required for TearScience® LipiFlow® treatment6
- Long-lasting results—many can see the continued benefit up to 12 months6
- 400,000 treatments worldwide—and growing7
Just 1 treatment increases mean gland secretion 3-fold and reduces more than 50% dry eye symptoms.8
Treatment also increased patient comfortable contact lens wear time by approximately 4 hours on average per day.9
TearScience® LipiFlow® treatment prior to cataract surgery improves mean dry eye symptoms and vision-related function scores post-surgery.10
Choose TearScience® LipiFlow®.
Because Eye Health Starts at the Surface.
REFERENCES:
- Craig JP, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017 Jul; 15(3): 276-283
- Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478.
- Xiao J et al. Functional and Morphological Evaluation of Meibomian Glands in the Assessment of Meibomian Gland Dysfunction Subtype and severity. Am J Ophthalmol. 2020;209:160-67.; Geerling G et al. Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction – Proceedings of the OCEAN group meeting. The Ocular Surface. 2017;15:179-92; Ye F,at el. Objective assessment of tear-film quality dynamics in patients with meibomian gland dysfunction and aqueous-deficient dry eye optical quality changes in different dry eye subtypes. Indian Journal of Ophthalmology. 2019;67(5):599-603
- Lane SS et al. A New System, the TearScience® LipiFlow®, for the Treatment of Meibomian Gland Dysfunction (MGD). Cornea. 2012;31(4):396-404. For illustrative purposes only. This content is presented for educational purposes only. JJSV Surgical Vision, Inc. does not engage in the practice of medicine and any clinical tips within this content are not a substitute for appropriate medical education and training or for the exercise of independent medical judgment. Each medical situation should be considered unique to each patient and all treatments individualized accordingly based on the respective physician’s medical judgment. JJSV Surgical Vision, Inc. does not (1) warranty the accuracy or completeness of any of the clinical tips, or (2) endorse or recommend any technique.
- Blackie CA, Coleman CA, Holland EJ. The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clin Ophthalmol. 2016; 10: 1385-1396
- TearScience® LipiFlow® IFU United States
- Data on file.
- Blackie CA et al. The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clin Ophthalmol. 2016; 10: 1385-1396.
- Blackie CA, et al. A single vectored thermal pulsation treatment for meibomian gland dysfunction increases mean comfortable contact lens wearing time by approximately 4 hours per day. Clin Ophthalmol. 2018;12:169-183.
- Data on file, 2018 (NCT01808560). Pilot study for treatment of meibomian gland dysfunction (MGD) prior to cataract surgery. Accessed from: https://clinicaltrials.gov/show/NCT1808560.
Content of courtesy of Johnson & Johnson Surgical Vision Inc.
Indications and important safety information for TearScience® LipiFlow® Thermal Pulsation System
Caution: Federal USA law restricts this device to sale by or on the order of a physician.
Indications: The TearScience® LipiFlow® Thermal Pulsation System is intended for the application of localized heat and pressure therapy in adult patients with chronic cystic conditions of the eyelids, including meibomian gland dysfunction (MGD), also known as evaporative dry eye or lipid deficiency dry eye.
Contraindications: Do not use the TearScience® LipiFlow® System in patients with the following conditions. Use of the device in patients with these conditions may cause injury. Safety and effectiveness of the device have not been studied in patients with these conditions. Ocular surgery within prior 3 months, including intraocular, oculo-plastic, corneal or refractive surgery procedure. Ocular injury within prior 3 months. Ocular herpes of eye or eyelid within prior 3 months. Active ocular infection (e.g., viral, bacterial, mycobacterial, protozoan, or fungal infection of the cornea, conjunctiva, lacrimal gland, lacrimal sac, or eyelids including a hordeolum or stye). Active ocular inflammation or history of chronic, recurrent ocular inflammation within prior 3 months (e.g., retinitis, macular inflammation, choroiditis, uveitis, iritis, scleritis, episcleritis, keratitis). Eyelid abnormalities that affect lid function (e.g., entropion, ectropion, tumor, edema, blepharospasm, lagophthalmos, severe trichiasis, severe ptosis). Ocular surface abnormality that may compromise corneal integrity (e.g., prior chemical burn, recurrent corneal erosion, corneal epithelial defect, Grade 3 corneal fluorescein staining, or map dot fingerprint dystrophy).
Precautions: The Activator or Activator II (Disposable) may not fit all eyes, such as eyes with small palpebral fornices. Use of the TearScience® LipiFlow® System in patients with the following conditions may result in reduced treatment effectiveness because these conditions may cause ocular symptoms unrelated to cystic meibomian glands and require other medical management. Safety and effectiveness of the device have not been studied in patients with these conditions. Moderate to severe (Grade 2-4) allergic, vernal or giant papillary conjunctivitis. Severe (Grade 3 or 4) eyelid inflammation (e.g., blepharochalasis, staphylococcal blepharitis or seborrheic blepharitis). Patients with severe eyelid inflammation should be treated medically prior to device use. Systemic disease conditions that cause dry eye (e.g., Stevens-Johnson syndrome, vitamin A deficiency, rheumatoid arthritis, Wegener’s granulomatosis, sarcoidosis, leukemia, Riley-Day syndrome, systemic lupus erythematosus, Sjögren’s syndrome). Taking medications known to cause dryness (e.g., isotretinoin (Accutane®) and systemic antihistamines). Esthetic eyelid and eyelash procedures (e.g., blepharoplasty, lash extensions, eyelid tattooing). In addition, the treatment procedure may loosen previously inserted punctal plugs, which may worsen the patient’s dry eye symptoms.
Adverse Effects: Potential adverse effects that may occur as a result of the procedure include, but are not limited to, the onset or increase in: Eyelid/eye pain requiring discontinuation of the treatment procedure; Eyelid irritation or inflammation (e.g., edema, bruising, blood blister, dermatitis, hordeolum or chalazion); Ocular surface irritation or inflammation (e.g., corneal abrasion, conjunctival edema or conjunctival injection (hyperemia)); and Ocular symptoms (e.g., burning, stinging, tearing, itching, discharge, redness, foreign body sensation, visual disturbance, sensitivity to light). Potential serious adverse events (defined as permanent impairment or damage to a body structure or function or necessitates medical or surgical intervention to preclude permanent impairment or damage to a body structure or function) that are not anticipated because of the device mitigations to prevent occurrence include: Thermal injury to the eyelid or eye, including conjunctiva, cornea or lens; Physical pressure-induced injury to the eyelid; and Ocular surface (corneal) infection.
ATTENTION: Reference the TearScience® LipiFlow® Thermal Pulsation System Instructions for Use for a complete listing of indications, warnings, and precautions.